RJ Lee Group 8 min read
Iron Sulfides in Concrete
Contributors
RJ Lee Group
Subscribe to our newsletter
Oxidation of Iron Sulfide Minerals
Iron sulfides are accessory minerals found in a variety of rock types, with pyrite (FeS2) and pyrrhotite (Fe1-xS) (x = 0 to 0.2), being most common. Under some conditions, these minerals can undergo oxidation reactions that have the potential to cause damage to concrete pavement or structures.
Pyrite in Rock Beneath Concrete Structures

Pyrite is common in shale and is a source of acid rock drainage (ARD), which occurs naturally in some environments as part of rock weathering and subsequent oxidation of the pyrite. This phenomenon is also known as acid mine drainage (AMD) when mining activities exacerbate the weathering process by exposing more pyrite to oxidation. ARD and AMD result in a lowering of the pH of ground and surface water flowing away from the pyrite source due to the release of sulfuric acid.
If pyritic shale or iron sulfide-bearing rock are present beneath buildings or pavements, the volume change caused by the oxidation of iron can result in swelling of the soil and subsequent heaving of concrete slabs and foundations1. In addition, the released sulfuric acid can react with calcite and other ions present to form expansive secondary sulfate minerals. Testing of shale for sulfide content is commonly performed when construction is planned, with limits varying by state and developer specifications.
Pyrite and Pyrrhotite in Concrete Aggregate
Iron sulfides are common in a variety of rock types and therefore may be present in aggregates used in concrete. Pyrite is the most stable iron sulfide mineral, but can still oxidize near the surface of concrete and stucco, leading to unsightly staining and pop-outs. In good-quality dense concrete, aggregate containing small amounts of pyrite can be successfully used without affecting the durability of the structure.

Pyrrhotite oxidizes more readily in concrete than pyrite. Reactions between the resulting sulfuric acid and the cement paste may form secondary sulfate minerals causing significant expansion and cracking of the concrete. In the most severe cases, alteration of the cement paste results in weakening and loss of structural integrity of the concrete. The use of pyrrhotite-bearing aggregates has been found to be the root cause of extensive damage to thousands of homes and commercial buildings in Eastern Connecticut2 and Quebec, Canada3-4.
A number of factors affect the rate and potential for damage due to the oxidation of pyrrhotite, including the concentration of the mineral, the concrete quality, the crystallographic mineral form, association with other iron sulfides, host rock mineralogy5, and the environment. The role of these factors and their effects on oxidation rate are not fully understood.
Thus far, consensus limits on the amount of pyrrhotite or any iron sulfide allowed in concrete aggregate have not yet been established in the United States. European standards for concrete aggregate have placed a limit of 1% total sulfur by mass, which is reduced to 0.1% total sulfur if pyrrhotite is identified in the aggregate.
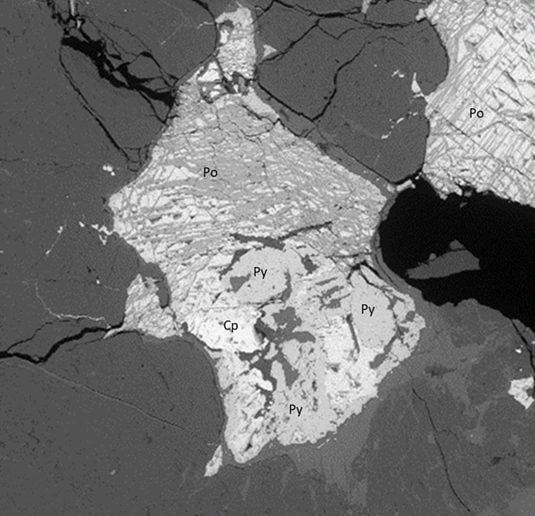
Researchers have recently developed a three-phase procedure to evaluate aggregates for potential deleterious reactions due to the oxidation of iron sulfides6. The procedure involves measuring total sulfur by LECO induction furnace, measuring oxygen consumption, and an accelerated mortar bar expansion test. Validation of this research is ongoing and will be coupled with new research efforts to identify appropriate limits for iron sulfide minerals in concrete aggregates to protect against future property damage while still permitting the use of aggregates containing non-deleterious quantities of iron sulfide minerals for concrete construction.
Citations:
1. Grattan-Bellew, P.E. and Eden, W.J. (1975). “Concrete Deterioration and Floor Heave Due to Biogeochemical Weathering of Underlying Shale,” Canadian Geotechnical Journal, Vol. 12, pp. 372–378.
2. Wille, K. and Zhong, R. 2016. “Investigating the deterioration of basement walls made of concrete in CT,” University of Connecticut, Storrs, CT. 93 p.
3. Chinchón, J. S., Ayora, C., Aguado, A. and Guirado, F. (1995). “Influence of weathering of iron sulfides contained in aggregates on concrete durability,” Cement and Concrete Research, Vol. 25, No. 6, pp. 1264–1272.
4. Rodrigues, A., Duchesne, J., Fournier, B. et al. 2012. “Mineralogical and chemical assessment of concrete damaged by oxidation of sulphide-bearing aggregates,” Cement and Concrete Research, ROI 42: pp. 1336-47.
5. Oliveira, I., Cavalaro, S.H.P., Aguado, A.(2014). “Evolution of pyrrhotite oxidation in aggregates for concrete,” Materiales De Construccion, Vo. 64, Issue 316, e038.
6. Rodrigues, A., Duchesne, J., Fournier, B., Durand, B., Shehata, M., Rivard, P. 2016. “Evaluation protocol for concrete aggregates containing iron sulfide minerals,” ACI Materials Journal, 113 (3): pp. 349-359.
Referenced Standards
ASTM C 33-18 Standard Specification for Concrete Aggregate
EN-12 620 (2003) Aggregates for concrete.
Applicable Services
LECO Carbon and Sulfur Analysis
X-ray Diffraction
Petrographic Examination of Aggregates for Concrete ASTM C295
Concrete Petrography ASTM C856
SEM/EDS of Concrete C1723
ICP-MS
Related Project Examples
Expansive Soils Investigations
Stucco Staining
Concrete Sidewalk Staining
Sulfide Sulfur in Shale
Concrete Petrography
Aggregate Testing
This summary description is offered for educational purposes only and no warranty (express or implied) is given on the applicability of this information to any particular part, material, application, or product. This article is not intended to be a complete dissertation on this topic and the reader is encouraged to contact RJ Lee Group for consultation regarding individual applications, materials, or situations.
Share this post


